UCLA researchers have shown in preclinical studies that their mass-producible engineered invariant natural killer T (iNKT) cells demonstrate promising antitumor efficacy and low immunogenicity (unwanted immune response) compared to current cell-based immunotherapy for cancer treatment.
Invariant natural killer T (iNKT) cells are specialized T cells that are notable for their speedy response to danger signals and activation of macrophages (white blood cells that destroy cancer cells, microbes, cellular debris, and foreign substances).
Dr. Lili Yang’s UCLA lab generated iNKT cells by engineering hematopoietic stem cells (HSCs, precursors to all types of blood cells). These cells are allogeneic—they are not genetically specific to patients. Normally, in the realm of cell-based immunotherapy, this would be expected to cause an immune response in the form of graft-versus-host disease (GvHD), a condition in which donor stem cells attack the recipient. The study mentioned that such immunogenicity can also decrease efficacy of therapeutic cells. Therefore, allogeneic cells have not been widely used for T-cell-based therapies, with most therapies using autologous (from the patient) cells instead.
Generally, autologous T-cell therapy requires a patient’s T cells to be extracted from blood, sent to a lab, engineered to find and kill cancer cells, then returned intravenously to the patient—all costing hundreds of thousands of dollars.
Unexpectedly, when tested on mice, the Yang Engineering Immunity Lab’s allogeneic HSC-iNKT cells did not cause the negative effects associated with allogeneic cells. The researchers found that while other types of allogeneic T cells killed mice by GvHD after 2 months of cell transfer, the mice that received HSC-iNKT cells sustained long-term survival.
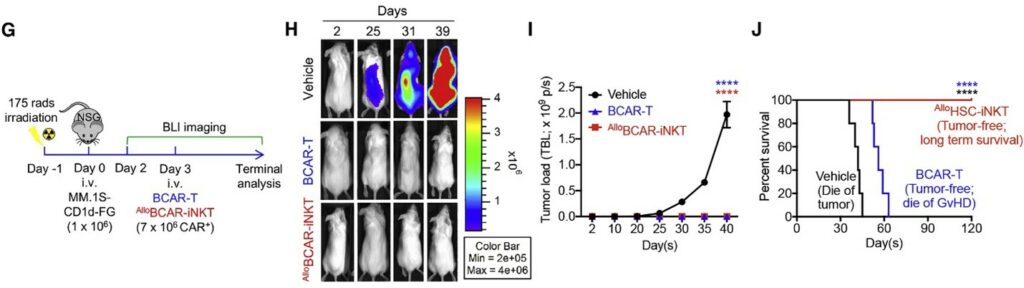
Following irradiation of mice, those without cell therapy (labeled as vehicle) died of tumors within 45 days. Those treated with allogeneic BCAR-T cells were tumor-free but died of GvHD. Only those treated with allogeneic HSC-iNKT were tumor-free and survived long term.
This important development means that cell-based cancer therapies would no longer have to rely only on autologous cells extracted from each individual patient. Instead, with the advent of the Yang lab’s one-size-fits-all allogeneic solution, therapeutic cells could be mass-produced and given to any patient, significantly bringing down treatment costs.
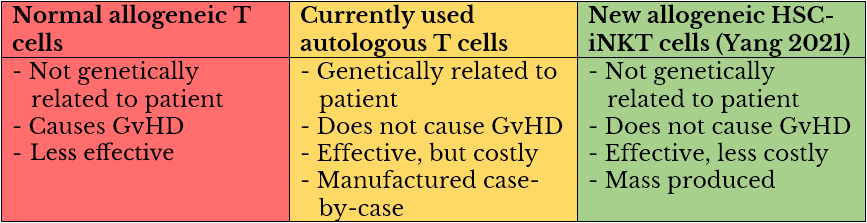
The reason why allogeneic HSC-iNKT cells do not cause GvHD is currently unknown to researchers.

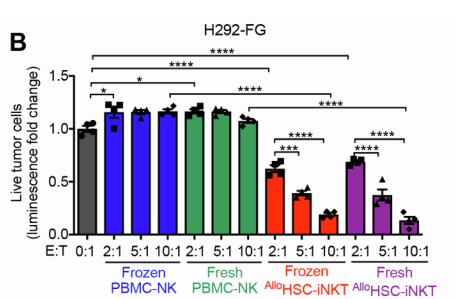
The study also showed that both frozen and fresh allogeneic HSC-iNKT cells killed live leukemia, melanoma, lung cancer, prostate cancer, and multiple myeloma cells in vitro. Compared to PBMC-NK cells, the Yang lab’s cells displayed greater tumor-killing efficacy. Importantly, allogeneic HSC-iNKT cells were also found to remain functional following freezing and thawing, which is crucial for their viability as a widespread, mass-produced treatment.
Factors that support allogeneic HSC-iNKT cells’ prospects as a future widespread cancer therapy include remaining functional following freezing and thawing, high tumor-killing efficacy, and mass-producible by virtue of low immunogenicity.
Dr. Yang told the UCLA Newsroom that one peripheral blood donation could yield 300,000 doses. The researchers are now focused on streamlining manufacturing processes, hoping to better enable mass-production, potentially bringing it to clinical and commercial development more quickly. The Newsroom noted that clinical trials have not yet occurred—this therapy has yet to be tested in humans or evaluated by the FDA. The UCLA Technology Development Group has filed a patent application for this method.
This article is based on the following sources
– UCLA scientists make strides toward an ‘off-the-shelf’ immune cell therapy for cancer. (2021, November 16). UCLA Newsroom. https://newsroom.ucla.edu/releases/off-the-shelf-immune-cell-therapy-for-cancer
– Yang, L., et al. (2021, November 16). Development of allogeneic HSC-engineered iNKT cells for off-the-shelf cancer immunotherapy. Cell Reports Medicine. https://doi.org/10.1016/j.xcrm.2021.100449
Reed is a Health Science student and published virology researcher at the University of Florida. His areas of interest are immunology and general biomedical research. Reed founded OneResearch.org as a free online source to highlight biomedical research and combat medical disinformation.