Introduction
Antimicrobial resistance (AMR) occurs when bacteria, viruses, fungi, and parasites evolve to become drug-resistant, pushing antibiotics and other antimicrobial medicines to practical obsoletism and thus increasing the difficulty of infection treatment. The prevalence of AMR is a pressing issue, cited by the World Health Organization (WHO) as one of the top 10 global public health threats facing humanity.
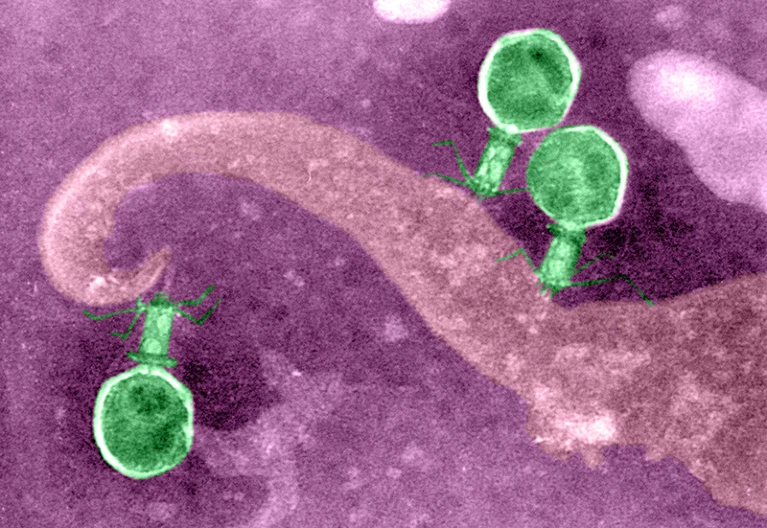
A promising threat to AMR is the use of “phage therapy”, a method of utilizing bacteriophages (phages) to eliminate specific bacterial strains.
Phages are viruses of bacteria that evolve to be extremely specialized, targeting only their certain type of bacterial cells and not affecting human cells. This opens a door for phage therapy to be used within the human body against bacterial infections, rather than solely antibiotics.
In the 1940s, the Western world saw chemical-based small-molecule antibiotics usurp potential research into phage therapies. Continued research into the potential of phage therapy allowed members of the former USSR to harness bacteriophages to treat disease. Phage therapy is now commonplace in Eastern Europe, specifically in Russia and Georgia where it is offered over-the-counter.
Dependency upon antibiotics has resulted in a fight against antimicrobial resistance, however, phage therapy may just be the method to bring us out of it.
Advantages of Phage Therapy
Bacteriophages occur abundantly on Earth, from the water of the oceans to the human GI tract. To attack bacterial cells, phages initiate the lytic cycle, wherein they first puncture the bacterium and then inject their own genetic information. The cell becomes a metaphorical warehouse for new phages, producing and assembling phages until the cell is full. Phages within the cell release endolysin, an enzyme that signals the end of the lytic cycle and causes the bacterial cell to explode, releasing brand-new phages.
There are many advantages to employing phage therapy over traditional antibiotic approaches.
Bactericide:
Phages are bactericidal, meaning they kill their host bacteria. However, some antibiotics are only bacteriostatic and cause a bacterium to become stuck within its stationary phase of growth rather than killing it, leaving behind the viable cell and opening a pathway for potential AMR.
Dosage:
As phages undergo the lytic cycle they self-amplify in an exponential fashion, which could potentially allow for a phage therapy to be effective with single or very low dosages. Lingering in the body after infection is not a worry, as phages only continue to multiply as they are killing bacteria.
Flora Bacteria:
Normal flora bacteria, “good” bacteria of the body, do not generally fall into the extremely specific target ranges of phages, which means they are almost totally unaffected by their presence in the human body. When antibiotics are introduced into the body, however, they tend to wreak havoc on any bacterium they come into contact with, including the normal flora that inhabits our bodies and aids us in many everyday functions.
Resistance:
When bacteria develop resistance to a chemical-based antibiotic it resembles an en masse revolt against one “villain”. Yet, bacteria find it relatively more difficult to develop resistance against phages due to their specific host ranges, since there is no single “villain”. Additionally, as bacteria evolve against phages, the phages have the capability to evolve right along with them, protecting the body against any bacterial strains that develop resistance.
FDA Approval
While phage therapy seems a promising opportunity, the US Food and Drug Administration (FDA) agency has yet to approve any therapies. As phage therapies are classified as biological products by the FDA, any manufacturing needs to adhere to standards like GMP, preclinical research, and all stages of clinical trials. This approval process is a tedious effort, however, as of October 2023 the US has the most phage-related Industry-Sponsored Trials (ISTs) on the worldwide scale, showing there is significant industry-related innovation on this front. Some examples of promising phage therapies in clinical trials within the US include WPP-201 for chronic venous leg ulcers and PreforPro for gastrointestinal distress.
Some of these ISTs are in their third phase of clinical trials, wherein the phage therapies are being tested for large-scale efficiency and comparison to currently market-available competitors. This is the final stage of testing prior to a drug or treatment to be submitted to the FDA for approval.
Despite the many advantages of phage therapy, there is still room for major disadvantages that are avoided through thorough testing prior to FDA approval. Namely, phages possess a narrow host range, inherent biological nature, and unfamiliarity within traditional Western medicine. For these reasons, along with the lengthy approval process, there is still time to go before Americans see phage therapies available to them for treatment.
Although the 1940s saw a shift away from bacteriophage research within Western countries, as time progresses, they could still very well be our savior from the self-inflicted antimicrobial resistance crisis.
References
Carroll-Portillo, A., & Lin, H. C. (2019). Bacteriophage and the innate immune system: Access and signaling. Microorganisms, 7(12), 625. https://doi.org/10.3390/microorganisms7120625
Durr, H. A., & Leipzig, N. D. (2023). Advancements in bacteriophage therapies and delivery for bacterial infection. Materials Advances, 4(5), 1249–1257. https://doi.org/10.1039/d2ma00980c
Furfaro, L. L., Payne, M. S., and Chang, B. J. (2018). Bacteriophage therapy: clinical trials and regulatory hurdles. Front. Cell. Infect. Microbiol. 8:376. doi: 10.3389/fcimb.2018.00376
Ledford, H. (2023, June 26). Why phage viruses could be the key to treating deadly infections – if they can be harnessed safely. Nature News. https://www.nature.com/articles/d41586-023-01982-2
Loc-Carrillo, C., & Abedon, S. T. (2011). Pros and cons of phage therapy. Bacteriophage, 1(2), 111–114. https://doi.org/10.4161/bact.1.2.14590
Strathdee, S. A., Hatfull, G. F., Mutalik, V. K., & Schooley, R. T. (2023). Phage therapy: From biological mechanisms to future directions. Cell, 186(1), 17–31. https://doi.org/10.1016/j.cell.2022.11.017
World Health Organization. (n.d.). Antimicrobial resistance. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
Yang, Q., Le, S., Zhu, T., & Wu, N. (2023, September 6). Regulations of phage therapy across the world. Frontiers. https://www.frontiersin.org/articles/10.3389/fmicb.2023.1250848/full#:~:text=While%20there%20is%20no%20FDA,clinical%20trial%20(Table%201).
Jordan is a Chemical Engineering student and undergraduate researcher at the University of Florida. She is passionate about genetic engineering and science communication. Her goal is to portray scientific information in a way that is understandable and beneficial to everyone, whether they are immersed in the scientific community or not.
