A team of surgeons at Duke University led by Dr. Carmelo Milano and Dr. Jacob Schroder were the first to implant a new generation of Total Artificial Heart (TAH) in a 39-year-old male patient with heart failure after receiving FDA approval for human trials.

CARMAT’s Total Artifical Heart product, called Aeson, is a new TAH that solves many of the issues with current treatment options associated with biventricular heart failure. Current options include human organ transplantation, which carries high risk of rejection. Such rejection can lead to the need for repeat cardiac transplant or even death. Other treatment options such as biventricular assist devices (BiVADs) carry high risk of neurological complication incidence such as stroke caused by accumulation of clots or seizures that can lead to life debilitating changes.
CARMAT’s Aeson is only the second TAH on the market. Aeson has made notable improvements over competitor SynCardia’s total artificial heart. CARMAT’s TAH is quieter and has a variable heart rate that adjusts based on patient activity, while SynCardia is notably louder and has a fixed heart rate.
Aeson replaces the patient’s heart by pumping blood to the pulmonary tract and systemic system. It does this through the use of battery-powered electrohydraulic rotary pumps with attached sensors that respond to changes in pressure and cardiac demands.
The French company’s device can be used as an intermediate heart prior to transplantation, or even as a complete replacement for living donor hearts. This development has radical implications to heart failure treatment, as American patients can expect to wait more than six months for a transplant.
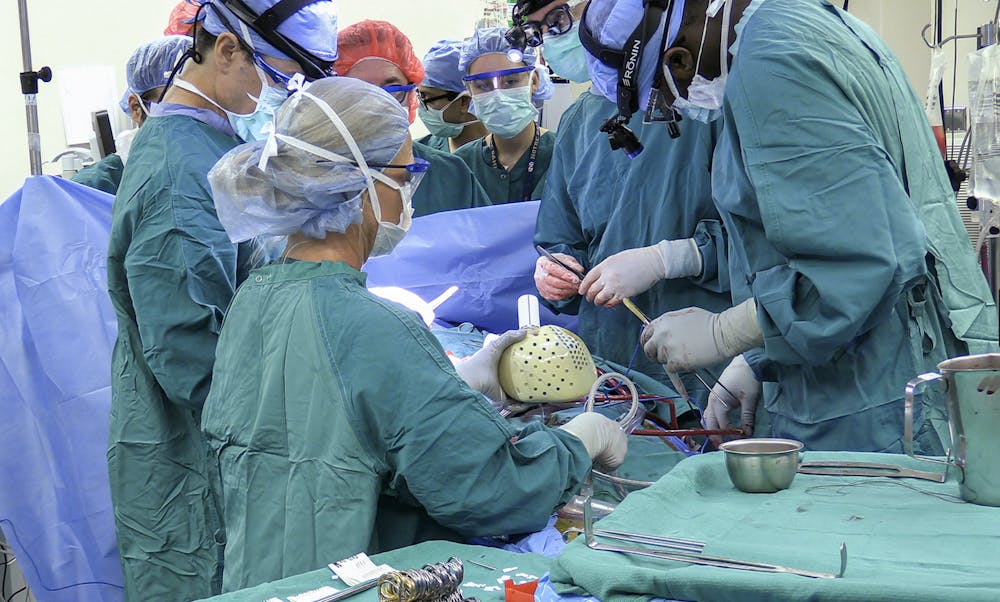
The surgery at Duke University was the first of its kind in the United States. It consisted of an 8-hour surgery in which Dr. Jacob Schroder and Dr. Carmelo Milano worked to remove Matthew Moore’s (a 39-year-old patient with biventricular heart failure on the transplant waiting list) left and right ventricles of his heart. They then installed the device successfully replacing the structures removed.
Each device costs around $190,000, not including the costs of critical care staff and other medications. However, this price point is significantly lower than the average cost for a human heart transplant, which is about $1.4 million.
CARMAT hopes to solve long wait lists and high costs with its TAH, Aeson, potentially offering a permanent solution in which a heart will always be available for any patient in need.
This article is based on the following sources
– Bailey, S. (2021, March 25). This new artificial heart responds to the patient. CNN Business. https://www.cnn.com/2021/03/25/business/carmat-artificial-heart-spc-intl/index.html
– Duke University School of Medicine. (2021, July 16). New generation artificial heart implanted in patient at Duke – First in U.S. https://medschool.duke.edu/blog/new-generation-artificial-heart-implanted-patient-duke-first-us
– Rapp, N., & Vandermey, A. (2017, September 14). Here’s what every organ in the body would cost to transplant. Fortune. https://fortune.com/2017/09/14/organ-transplant-cost/
– Tan, K. (2021, July 29). Duke surgical team successfully implants new generation artificial heart in patient, first in U.S. Duke Chronicle. https://www.dukechronicle.com/article/2021/07/duke-university-hospital-health-artificial-heart-transplant-research-study-carmat
– University of California, San Francisco Health. (n.d.). FAQ: Heart transplant. https://www.ucsfhealth.org/education/faq-heart-transplant
